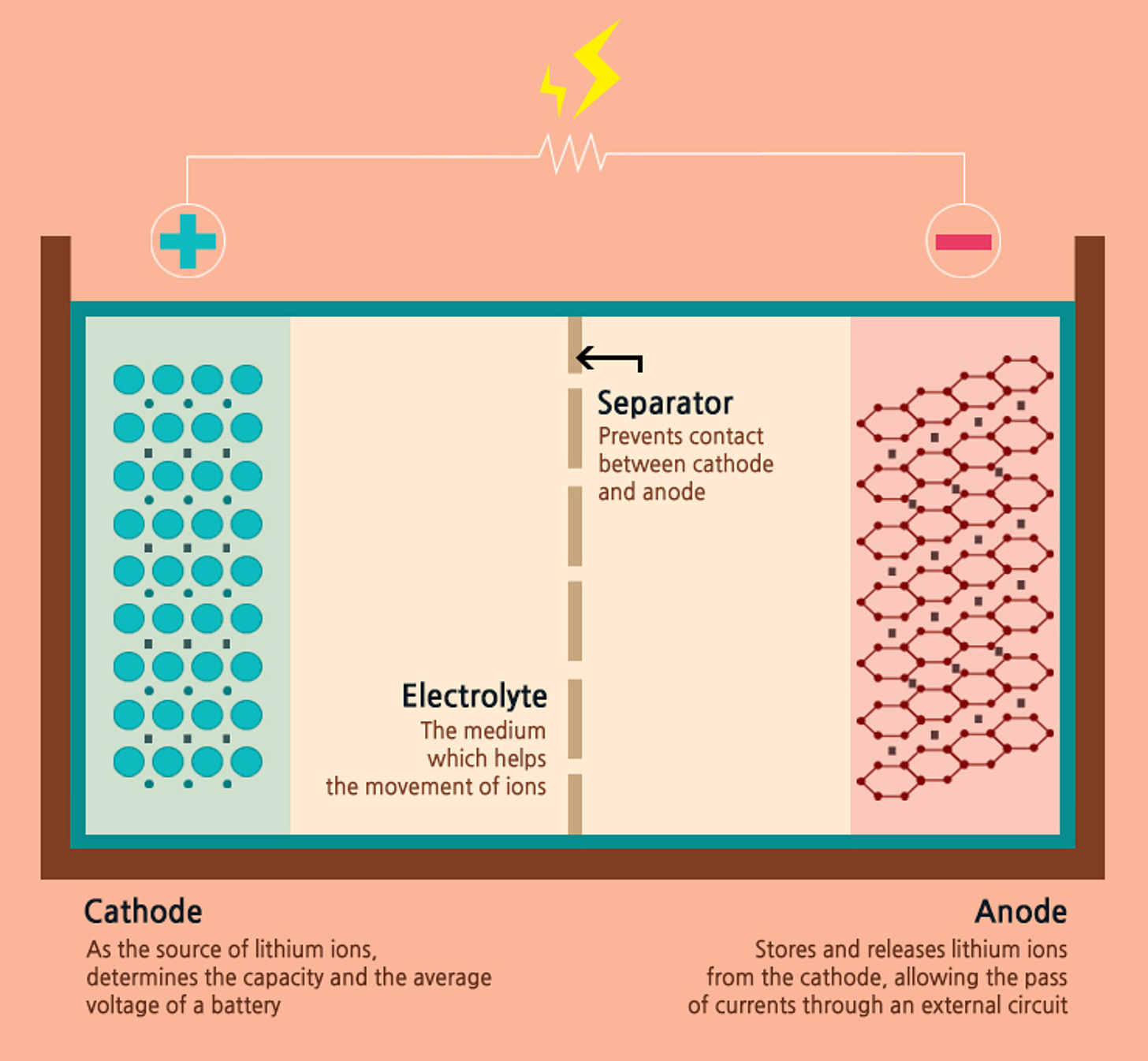
Figure 2: The Four Components of Li-ion Battery [2]
An Li-battery is typically made up of the following components[3]:
- Cathode: a “source of the Li-ions”, as it tends to lose electrons easily and turn into a positive ion. The cathode is crucial for determining the capacity of the and voltage of the battery, influencing its performance.
- Anode: acts as the 'storage' for the Li-ions released from the cathode. This movement of electrons from the cathode to the anode is the current, which is facilitated by an electrolyte.
- Electrolyte: The electrolyte must have high ionic conductivity for the smooth movement of lithium ions, as well as high electrochemical stability and high flash point for safety. Also, it is necessary to prevent the electrons from entering the electrolyte and make them only move along the external conducting wire.
- Separator: it prevents any physical contact between the cathode and anode, and the tiny pores on the separator allow lithium ions to move. The separator needs to have high electrical insulation and thermal stability for safety, and it must also automatically block the movement of ions at a temperature above a certain level.

